Topic 4: Observation of the Motions of Ion Permeation Pathways on Cell Surfaces at the Single-Molecule Level
Detection of the Precise and Dynamic Conformational Changes of Channel Molecules
Cells contain various kinds of pore-forming proteins, termed channel molecules, that penetrate the cell membrane. These channels regulate the flow of ions across the membrane by receiving various stimuli. The total number of channel molecules on the cell membrane of a single cell may reach a maximum of 200,000-300,000. Potassium channels are representative channel molecules through which only potassium ions can selectively flow. These proteins are found in cells of a wide variety of organisms, ranging from bacteria to humans. Various diseases, including sudden infant death syndrome, are caused by dysfunctions of ion channels. To contribute to the development of therapies for such diseases, many researchers around the world are studying the mechanisms of channel molecules around-the-clock. In particular, experiments conducted at SPring-8 have succeeded for the first time in visualizing the detailed real-time motion of the opening and closing of channel molecules. These findings have captured the world's attention.
“Video Images,” not Still Images, are Required
Potassium channel molecules quickly take in or block only potassium ions upon receiving stimuli. By what mechanisms do potassium channel molecules regulate the flow of potassium ions?
Dr. Erwin Neher, a German biophysicist, and Dr. Bert Sakmann, a German physiologist, established a technique for isolating a single ion channel molecule and measuring its electronic properties. For their achievements, they received the Nobel Prize in Physiology or Medicine in 1991.
Dr. Roderick MacKinnon, an American neurophysiologist, revealed the three-dimensional structures of potassium channel molecules, and received the Nobel Prize in Chemistry in 2003. However, only a few studies have been conducted to reveal the three-dimensional structures of protein molecules in biomembranes (membrane protein).
In October 2001, a project called “Research on in vivo dynamic structures and functions of proteins using diffracted X-ray tracking (DXT)” was initiated as part of the Core Research for Evolutional Science and Technology (CREST) project, funded by the Japan Science and Technology Agency (JST). Dr. Yuji Sasaki1) (Senior Scientist, JASRI) led a group to conduct this project. Also participating were Dr. Shigetoshi Oiki (Professor, Fukui University School of Medicine) and Dr. Hirofumi Shimizu (Assistant Professor, Fukui University School of Medicine). An important target of this group was to explore the dynamic structures of potassium channel molecules by detecting the real-time motion of potassium channel molecules.
1n 1997, Dr. Sasaki invented the “DXT method,” for which he received the IBM Japan Science Prize in 2007. In this method, a gold nanocrystal is first bound to a single potassium channel molecule. This gold nanocrystal that he developed is a layered crystal 20 nm (1 nm = 10-9 m) in thickness. Unlike conventional crystals, from which many diffraction spots are produced by the diffraction of X-rays, this crystal provides only one or two diffraction spots from each crystal. The crystal is exposed to X-rays, and the movement of the target molecule is determined from the movements of diffraction spots emitted from the gold crystal. Dr. Sasaki, an expert on measurement, sought applications of this method and found potassium channels. “The static structures of potassium channels had been determined, but their dynamic mechanisms had not been known at all. Therefore, I believed that the elucidation of their dynamic mechanisms would be highly impactful,” said Dr. Sasaki to explain the background of his research.
X-ray diffraction utilized in this experiment is a phenomenon through which rotary motions of diffraction spots can be monitored with an angular resolution of less than 1 milliradian. A 1-milliradian angle is equivalent to a 1-mm displacement of a diffraction spot when the distance from the sample increases by 1 m. This level of precision cannot be achieved in other diffraction experiments that use visible light. Moreover, a special X-ray source is required to measure the continuous motions of spots. Highly brilliant white X-rays produced at the RIKEN Structural Biology II beamline (BL44B2: currently renamed to the RIKEN Materials Science beamline and only monochromatic X-rays are available) were indispensable to this measurement. Unlike monochromatic X-rays, white X-rays contain various wavelengths of X-rays, from which a broad range of information about molecular motions can be obtained.
1) Currently Professor at the University of Tokyo, Japan.
Opening and Closing of the Potassium Channels are “Twisting Motions”
This experiment was conducted according to the procedure explained in Fig. 1. Two kinds of samples, whole molecules and molecules without intracellular regions, were provided from Dr. Oiki for the purpose of observing the opening/closing positions of channels. First, potassium channel molecules were attached to a glass plate outside the cell, and a gold nanocrystal was bound to the inside of the cell. Highly brilliant white X-rays were shined on the potassium channel molecules, and diffraction spots emitted from the gold nanocrystals were detected with high precision using an X-ray detection monitor. As gold nanocrystals moved, bright spots corresponding to diffraction spots moved on the monitor. Since the gold nanocrystals are bound to the channel molecule, the conformational changes of the channel can be observed as motions of bright spots on the monitor. Deformation of the channel can be observed as motion in the radial direction, and twisting motion can be observed as movement in the circumferential direction.
A passageway for ions is located at the center of the potassium channel molecule (Figs. 1B and 2C). Which functions of potassium channel molecules are responsible for opening/closing of this ion passage? Answering this question is the aim of this experiment.
The data revealed that the answer is “twisting motion” (Fig. 2). When the channel molecule was being closed, only radial motions were observed, indicating that the channel molecule is slightly bent. However, notable twisting motions of the channel molecules were observed when opening and closing were repeated. Moreover, conformational changes in the opposite direction were observed when the channel was closing. These findings indicate that the intracellular aperture of the channel is closed and opened according to the twisting motions of the channel molecule. Thus, the secret of the opening and closing of channel molecules has been revealed for the first time.
This potassium channel molecule has a structure that extends into the cytoplasm (Fig. 1B right). Similar twisting motions were observed in the measurement of the opening and closing of the channel molecule by binding a gold nanocrystal to the end of this extended domain. This finding indicates that the twisting motions are extended all the way to the end of the molecule, within the cytoplasm.
More interestingly, the mechanisms of action have been determined for therapeutic agents, “channel inhibitors,” used to treat diseases in which channel protein molecules are involved. The molecules of these agents reach deep into the pores of the channel molecules, where ions are passing, and block ions by occluding the pore. Indeed, the study of conformational changes revealed that the twisting motions were halted. This finding reveals that the channel inhibitors not only block the flow of ions but also fix the conformations of the channel molecules. Furthermore, it reflects the fact that the conformational changes of the channel molecules take place near the pore.
These research achievements were published in Cell (January 2008) and highly commended. One writer commented, “This observation of single-molecule conformational changes has made a significant mark on the history of channel research.”
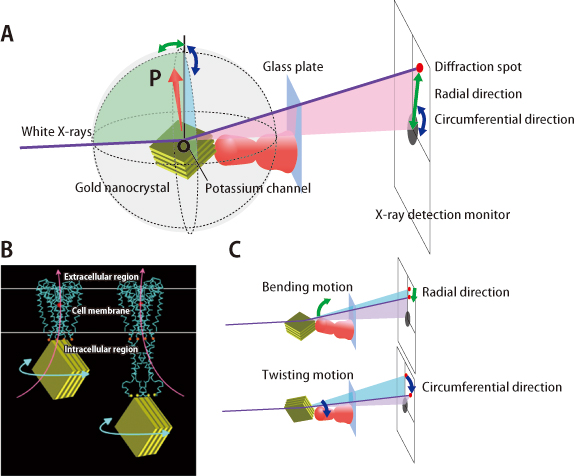
A: A gold nanocrystal is attached to a channel molecule that is fixed on a glass plate. Upon irradiation with X-rays, diffraction spots emitted from the gold nanocrystal are observed on an X-ray monitor. An arrow (OP) indicates the direction of the X-ray diffraction plane of the gold nanocrystal. B: Observation of potassium channel motions under two conditions. By using two types of molecules (whole molecules and those without intracellular regions), the opening/closing regions of the channel were directly examined in order to identify the locations where the motions were initiated. The gold nanocrystals were attached on the intracellular side of the channels. Motions were examined just below the cell membranes (left) and at the end of the channel on the intracellular side (right). Red circles indicate the locations where channel inhibitors bind. Orange and yellow circles represent the binding locations with the gold nanocrystals. C: Correlation between conformational changes of the channels and the diffraction spots. Diffraction spots move in the radial direction when the channels bend, and in the circumferential direction when the channels twist.
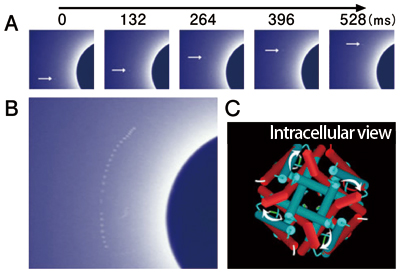
A: Sequential images of the movements of diffraction spots. Actual images were taken every 33 ms, but only selected images are shown. B: Trajectory of diffraction spots. Images taken every 759 ms are overlaid. A blue circle found on the right-hand side corresponds to a black circle located in the center of the X-ray monitor in Fig. 1A. The circular motion of the diffraction spots indicates the twisting motion of the channel. C: The conformational changes of the channel upon opening and closing, viewed from the intracellular side. The ion aperture is open as the state changes from blue (closed state) to red (open state).
Capturing the Changes in Channel Molecules at Microsecond Timescales
Channel molecules exist ubiquitously in all cells of all organisms. Dysfunctions of ion channels cause various “channel diseases” such as sudden infant death syndrome, heart attack during exercise in the young, and sudden death from an unknown cause during sleeping. Epilepsy is also believed to be caused by dysfunctions of ion channels. Thus, this research can offer highly promising clues regarding the dynamic mechanisms of dysfunctions of ion channel molecules in these patients. Once new concepts of drug development are established based on the dynamics of molecules, it will be possible to obtain revolutionary therapeutic drugs that exert dramatic effects.
“Our next target is to simultaneously observe the conformational changes of ion channels at the single-molecule level. At SPring-8, real-time measurement techniques at the microsecond level have been already established, so that simultaneous observations will become possible in the not-so-distant future,” mentioned Dr. Sasaki to explain future prospects. This project has been appointed as one of the SPring-8 Core Research Projects, and experiments will continue until 2011. A related new CREST research project is also ongoing.
Reference
1. H. Shimizu, M. Iwamoto, T. Konno, A. Nihei, Y. C. Sasaki and S. Oiki; Cell, 132, 67 (2008)