Clarification of Structure of a Complex That Plays Central Role in Photosynthesis - A major step toward artificial photosynthesis
We can live thanks to photosynthesis
Japanese students learn about photosynthesis at elementary school. Although you may wonder if there remain unresolved mysteries on photosynthesis, there are indeed still many mysteries that are not solved. In addition, the clues to solving environmental and energy problems may be hidden behind these unresolved mysteries.
Professor Jian-Ren Shen of Okayama University, who has studied photosynthesis for over 20 years, said, “People do not understand how important photosynthesis is for us.” All of the oxygen on earth is generated by photosynthetic organisms, such as plants. The amount of oxygen produced per year is approximately 260 billion tons. The total amount of oxygen on earth is approximately 1.2 quadrillion tons, meaning that all of the oxygen on earth is circulated in approximately 4,600 years. “This span of time is not long compared with the history of evolution,” says Professor Shen. The organisms on earth exist while maintaining a delicate balance between the amount of oxygen produced by photosynthetic organisms and that consumed by other organisms including humans.
Photosynthesis is the only way to convert solar energy to the energy of organic substances that can be used by animals and plants. Through photosyntehsis, plants produce organic substances that are used as sources of nutrients for their growth. Plants are eaten by herbivores, which are then eaten by carnivores. Through this food chain, animals including humans obtain the energy necessary for their survival from photosynthetic organisms. Fossil fuels, such as petroleum and coal, originate from preserved remains of animals and plants, which are different forms of organic substances produced by photosynthesis in ancient times. We completely depend on photosynthetic organisms for the supply of oxygen and organic substances on earth.
What is artificial photosynthesis?
Photosynthesis is a process through which carbohydrates (which are organic substances) and oxygen are produced using carbon dioxide and water. However, this is a summary description of various reactions of photosynthesis. Many reactions instead of only one are included in photosyntehsis.
The reactions are roughly divided into two pathways: the light reactions, in which chemical reactions are induced upon absorption of solar energy, and the dark reactions, in which carbohydrates are produced from carbon dioxide using the products of light reactions (Fig. 1).
In the first step of light reactions, water molecules are split to produce oxygen molecules, hydrogen ions, and electrons using solar energy. It is only in this step of photosynthesis that oxygen is produced. Namely, the oxygen produced in this step is the source of oxygen present in the atmosphere. The electrons released in this step are sequentially transferred from one protein to another (electron transfer) and finally stored in nicotinamide adenine dinucleotide phosphate (NADPH) (Fig. 2). The hydrogen ion gradient across the chloroplast membrane facilitates the production of adenosine triphosphate (ATP). “If we can retrieve electrons from water by artificially replicating the first step, the electrons can be used as electrical energy. Photosynthetic organisms use only 0.1% of the available solar energy on earth. We are aiming at the realization of artificial photosynthesis by which abundant solar energy can be converted into other forms of energy that humans can use,” says Professor Shen.

Fig. 1 Schematic of photosynthesis
In the first step of light reactions, water is split to produce oxygen, and ATP and NADPH are then stored. In the dark reactions, carbohydrates are produced from carbon dioxide using ATP and NADPH.

Fig. 2 Process of light reactions
In the first step of light reactions, water molecules (H2O) are split to produce oxygen molecules (O2), hydrogen ions (H+), and electrons (e-) using solar energy. The electrons are sequentially transferred from one protein to another and finally stored in NADPH. The hydrogen-ion gradient across the thylakoid membrane facilitates the production of ATP. The membrane proteins are in orange; they are all different proteins with different functions.
Longtime bottleneck
As a matter of fact, for many decades many researchers have focused their studies towards the realization of artificial photosynthesis. However, it has been a rough road for them, and today, many researchers are still tackling this problem.
Light irradiation of water alone can never induce water splitting. A catalyst to induce the reaction is required. In photosynthesis, photosystem II (PSII), a protein complex, functions as a catalyst. Thylakoid is a flat saclike structure contained in a chloroplast, in the membrane of which, PSII is embedded (Fig. 2).
In PSII, there is a “channel” through which water molecules are introduced, and at the end of this channel, there is a catalytic center where water splitting occurs. Upon the introduction of water molecules into the channel, PSII with catalytic centers changes its crystal structure to induce water splitting using solar energy. After this reaction, PSII returns to its original structure. If the changes in the crystal structure of the catalytic center in PSII are clarified in detail, we should be able to artificially synthesize chemical compounds with the same catalytic effect of PSII by mimicking its structure.
However, this is not an easy task. To examine the structure of a protein, it is necessary to prepare a sufficient amount of the protein, purify it, and form its crystals without lattice distortion. PSII is a giant protein complex consisting of 19 proteins and is embedded in the thylakoid membrane. It is extremely difficult to examine such a membrane protein complex, and researchers have faced a difficulty in producing PSII crystals without lattice distortion.
Finally determined crystal structure of PSII
Professor Shen extracted PSII from one type of cyanobacteria,*1 and examined various conditions for its crystallization. He first succeeded in the production of PSII crystals in 1999. However, their quality was not sufficiently good for clarifying their crystal structure in detail. After that, Professor Shen endeavored to improve crystal quality for another decade; finally, he succeeded in obtaining good-quality PSII crystals in 2009. As a result of the structural analysis of the obtained PSII crystals using X-rays at SPring-8, a clear image of the PSII crystal structure was obtained (Fig. 3).
Professor Nobuo Kamiya of Osaka City University, who carried out the structural analysis, says, “The resolutions of the images obtained using conventional PSII crystals were insufficient and only obscure structures were found. The resolutions of the images obtained using the crystals formed this time were significantly improved. So, we precisely determined the arrangement of atoms and distances between them. The information obtained from these crystals will be extremely valuable for the realization of artificial production of the catalytic centers of PSII.” He also added, “We have been working on the structural analysis of PSII for more than 20 years. Without SPring-8 X-rays, we would not have been able to obtain a clear crystal structure of PSII even if we had successfully produced good-quality PSII crystals. Two events, i.e., the completion of SPring-8 and the successful production of good-quality crystals, occurred simultaneously, which is one of the reasons for this achievement.
On the basis of the structural analysis results, it was found that the catalytic center of PSII consists of four manganese, one calcium, and five oxygen atoms, and four water molecules (Fig. 4). This is the first time in the world that the crystal structure of PSII has been clarified at this resolution. “The catalytic center of the PSII has a shape similar to a distorted chair. It is considered that the catalytic center can flexibly change its structure because of this flexible structure; thus, it can serve as a catalyst,” says Professor Kamiya.
A research group in Osaka City University to which Professor Kamiya belongs formulated a plan to develop an artificial photosynthesis system that produced methanol fuel and to put it into practical use by 2020. “The energy from electrons released by the water-splitting reaction should be stored. We plan to produce hydrogen using the electrons and hydrogen ions released through the water-splitting reaction, and to produce methanol using the thus-obtained hydrogen, as well as carbon dioxide and oxygen. However, much light should be collected to produce a large amount of methanol. We are planning to build a system for artificial photosynthesis by floating large panels on the sea. We still have many problems to overcome, but we believe that we can realize our plan,” says Professor Kamiya.
The realization of artificial photosynthesis will contribute to solving not only energy problems but also environmental problems because carbon dioxide, a cause of global warming, can be used as a material for methanol production. The realization of this dream is becoming more likely.

Fig. 3 Overall structure of PSII
PSII has a dimer structure composed of two monomers*2 each consisting of 19 proteins. There is a symmetrical axis at the center of PSII. Two red circles indicate the catalytic centers. The blue spheres indicate water molecules.
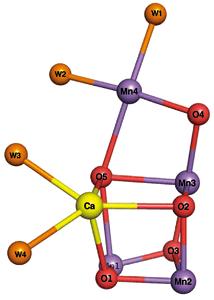
Fig.4 Structure of catalytic center in PSII
The catalytic center of the PSII has a shape similar to a distorted chair and consists of four manganese (Mn), one calcium (Ca), and five oxygen (O) atoms, and four water molecules (W).
Column : Crystals generated as a result of unrelenting effort
 |
| Professor Nobuo Kamiya (left) and Professor Jian-Ren Shen (right) |
Approximately 20 years ago, Professor Shen succeeded in the production of PSII-like crystals. However, Professor Kamiya thought that they were not crystals on the basis of the X-ray diffraction data he examined. Professor Kamiya recalls, “I can remember the face of Professor Shen, when I told him that they were not crystals. He was so disappointed that I irresponsibly told that ‘There is some possibility that they could be crystals.’”
The substances produced then were not crystals; however, Professor Shen continued to work on the production of PSII crystals in the face of many difficulties. “As I was doing my research, I didn’t know whether my effort would pay off. But I believed that someone should pursue this research,” says Professor Shen. It is impressive that the unrelenting effort of Professor Shen has led to this achievement and paved the way toward artificial photosynthesis.
Glossary
*1 Cyanobacteria
Cyanobacteria are prokaryotic organisms with non-nucleated cells. Cyanobacteria are estimated to have first appeared 2.7 billion years ago on earth and are considered to be the oldest oxygenic photosynthetic organisms.
*2 Monomer
A monomer is a building block of coupled multimeric complexes of identical proteins, such as dimers and trimers.
Interview and original text by Chisato Hata (Sci-Tech Communications Incorporated)
This article was written following an interview with Professor Jian-Ren Shen (Graduate School of Natural Science and Technology, Okayama University) and Professor Nobuo Kamiya (The Osaka City University Advanced Research Institute for Natural Science and Technology).