Topic 1: Structural Analysis of Membrane Proteins
Exploration of the Behavior of Proteins in Biological Membranes
A cell, the basic unit of life, is encapsulated by a phospholipid bilayer called the cell membrane. Subcellular organelles such as nuclei and mitochondria are also enclosed by phospholipid membranes. These membranes contain various membrane proteins that account for one-third of all types of proteins, and which function in diverse ways. For example, membrane proteins expel sodium ions from cells, and bring potassium ions in, upon neural excitation. Investigation of the structures and functions of these membrane proteins, which are embedded in cell membranes and difficult to handle, is extremely challenging and regarded as a major target in biology. SPring-8 has visualized the structures of many membrane proteins, in order to lead the advancement of medicine and pharmacology.
Success in the Visualization of the Structure of Rhodopsin
Rhodopsins, vision sensor molecules contained in the retina, receive visual signals that contain a great deal of information. Rhodopsins are a member of the guanine nucleotide-binding protein-coupled receptor (GPCR) family. GPCRs function in many contexts as ultra-high sensitive sensor membrane proteins, and are responsible for the successive activation of numerous functions by sensitively responding to subtle signals such as visual/olfactory stimuli or immune signals.
Dr. Masashi Miyano (Chief Scientist, RIKEN Harima Institute, Japan)1), Dr. Krzysztof Palczewski, (Professor, Washington University, the United States), and colleagues have been performing structural analyses of bovine rhodopsins. “About 80% of all GPCRs are similar in structure to rhodopsins, but structural analyses have not advanced far because crystallization of rhodopsins is difficult and the reproducibility of rhodopsin crystals is poor,” commented Dr. Miyano.
As expected, the crystallization of bovine rhodopsin was extremely difficult, but Dr. Tetsuji Okada (Washington University)2) succeeded after 5 years of dedicated research at RIKEN and Nagoya University, Japan. In the spring of 2000, Dr. Masaki Yamamoto (RIKEN)3) and colleagues conducted X-ray diffraction experiments to analyze a few tens of these crystals at the RIKEN Structural Biology I beamline (BL45XU), which is optimized for multi-wavelength anomalous diffraction (MAD). MAD is a wavelength-dependent measurement technique of X-rays scattered by atoms; the method allows us to analyze even a single crystal of protein by labeling it with mercury.
This research group determined the structures of rhodopsin and the vitamin A derivative retinal, which is complexed with rhodopsin (Fig. 1). Bovine rhodopsin has seven α-helices; an α-helix is a building block of protein consisting of amino acids in a spiral conformation. These seven α-helices penetrate the cell membrane; the last α-helix is shortened by a 90° bend. Many of the mutations that cause visual disorders have been identified as mutations of amino acids in the hydrogen bonding moieties that stabilize the gaps between these α-helices.
The results of these research findings were published in Science (August 2000) and received a highly commendable evaluation from the journal's reviewers as the most important paper in this research field in the last 15 years. This paper has been cited for more than 2,700 times, making it one of the most cited papers in the world.
Surprisingly, half of the drugs currently being developed are targeting GPCRs. These findings relate to the structure of rhodopsin, which is a representative GPCR, and is therefore expected to be a basis for drug development.
For this achievement, Dr. Miyano and Dr. Okada received the Prize for Science and Technology (Research Category) by the Minister of Education, Culture, Sports, Science and Technology of Japan in 2010.
1) Currently Professor at Aoyama Gakuin University, Japan.
2) Currently Professor at Gakushuin University, Japan.
3) Currently a director of the Basic Research Division, RIKEN.
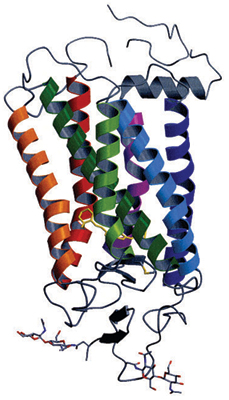
Seven α-helices penetrate the cell membrane, and there is a short helix that is bent by 90°. Yellow molecules indicate retinal.
Exploring the Structures of Calcium Pumps
Muscle cells contain bag-shaped sarcoplasmic reticula, which store calcium. Muscle fibers contract when calcium is released from the sarcoplasmic reticula, while they relax when calcium returns. Integration of these calcium transfer processes induces muscle motion. However, calcium concentration in the sarcoplasmic reticula is 10,000 times higher than the concentration outside, and thus it is easy for calcium to exit but difficult for it to return against the concentration gradient. The calcium pumps in the sarcoplasmic reticula are also membrane proteins. Calcium pumps are driven by the energy that is released when adenosine triphosphate (ATP) is hydrolyzed.
According to Dr. Chikashi Toyoshima (Professor, The University of Tokyo, Japan), he and his colleagues were conducting research on calcium pumps with the goal of clarifying the atomic structures of calcium pumps. In 1996, they succeeded in the crystallization of calcium pump proteins, for the first time in the world. They continued structural analyses of these crystals with high-performance electron microscopes. At the time, however, the resolution was limited to a level at which secondary structures, such as α- helices were barely observable; atomic resolution was not available.
SPring-8 started up around this time, and Dr. Toyoshima tried to analyze his crystals by applying X-ray diffraction techniques. In the autumn of 1998, he began his experiment on calcium pump crystals at the RIKEN Structural Biology I beamline (BL41XU). Although the thickness of the crystals was only several micrometers, not thick enough for X-ray analyses, he obtained potentially promising results from this first experiment. Therefore, he expected his goal would be achieved once thicker crystals became available. Since then, the quality of data had been improved as thicker crystals have been obtained.
In June 2000, they finally succeeded in collecting high-resolution diffraction data at 0.26 nm (1 nm = 10-9 m) to elucidate the 3D structures of pump proteins, both when they were coupled with calcium and when they were not. The detailed research results were reported in Nature (June 2000), and the world first 3D structure image of calcium pumps appeared on the cover of the journal.
Subsequently, in August 2002, this research group revealed the structures of calcium pumps after the transport of calcium. The calcium pump was found to consist of ten α-helices and three cytoplasmic domains (A, N, P). These α-helices are arranged in a configuration resembling the staves of a wooden barrel. One of the α-helices works like a piston to release the coupled calcium; the whole calcium pump moves extensively. No scientists had imagined that membrane pumps would work so similarly to a hand-pump.
Dr. Toyoshima and colleagues elucidated the four-stage mechanisms of calcium pumps (Fig. 2); the detailed results were published four times in Nature (June 2000, August 2002, June and November 2004). This achievement is comparable to the discovery of the sodium pumps with similar mechanisms, which were recognized with a Nobel Prize. Dr. Toyoshima received the Asahi Prize in 2009 for elucidating the mechanical operation of the calcium pump.
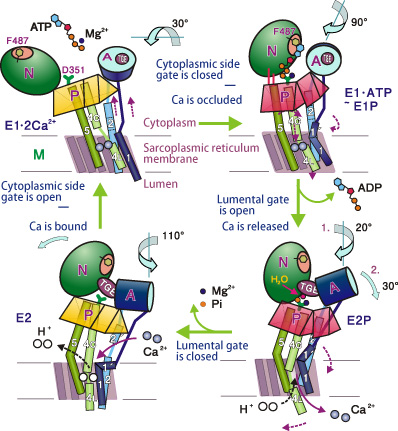
Bottom left: Three domains are standing in groups in the absence of calcium. Top left: Domains are open when calcium ions are bound with pump proteins. Top right: N-domain approaches the P-domain; ATP connects the N- and P-domains; and the A- and N-domains are also connected. The A-domain and M1-helix occlude the entrance to the pump. Bottom right: A-domain rotates; the M1-helix changes direction; the bottom half of the M4-helix changes direction; and the lumenal gate opens. Furthermore, the P-domain tilts, the M5-helix bends; and the M3- and M4-helices come down like a piston to eject calcium ions.
Gap Junction Channels that Interconnect Cells
Cells function by closely collaborating with each other. For example, heart muscle cells keep pace with each other in order to make the heart beat regularly. The key to this synchronization is the gap junction channel, which is also a membrane protein. The gap junction channel directly interconnects two cells like a bridge.
In 2007, a Kyoto University group found, by using electron microscopy, the structure of a closed gap junction channel. Subsequently, Dr. Tomitake Tsukihara 4)(Professor, The University of Hyogo, Japan) and colleagues discovered the structure of an open gap junction channel, through X-ray crystal structure analyses at SPring-8. “The gap junction channel has a shape similar to that of a Japanese hand drum, and has a 1.4-nm hole along the longer axis of the channel molecule. This hole allows small molecules and ions to go through the channel,” reported Dr. Tsukihara.
In the closed gap channel, there is a structure at the upper end that was speculated to block the channel. However, no such blocking structures were found on the channel in the open state; instead, six short α-helices formed the funnel-like structure (Fig. 3).
These research results were published in Nature (April 2009). More than 20 constituent proteins that construct gap junction channels have been discovered in humans. Variant proteins that have similar structures to these gap junction channel proteins are thought to cause many diseases; the exploration of their structures is expected to provide clues that will help us to develop effective therapies for these diseases.
4) Also Professor Emeritus at Osaka University, Japan.
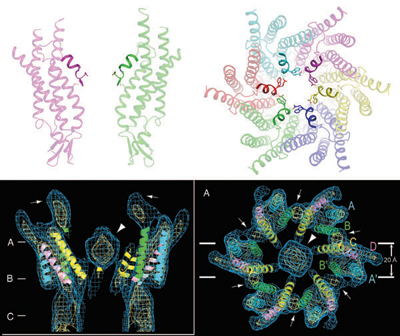
Sideview (left) and overhead view (right).
Six short α-helices that form a funnel-like structure have been identified at the upper part of the channel, but no structures block the channel path in the open state (top). In contrast, some structures can be seen in the center of the gap channel in the closed state; these structures are speculated to block the channel (Bottom: these figures are reproduced from A. Oshima et al., Proc. Natl. Acad. Sci. USA 2007 104(24)).
References
1. K. Palczewski, T. Kumasaka, T. Hori, C. A. Behnke, H. Motoshima, B. A. Fox, I. Le Trong, D. C. Teller, T. Okada, R. E. Stenkamp, M. Yamamoto and M. Miyano; Science, 289, 739 (2000)
2. C. Toyoshima, M. Nakasako, H. Nomura and H. Ogawa; Nature, 405, 647 (2000)
3. C. Toyoshima and H. Nomura; Nature, 418, 605 (2002)
4. C. Toyoshima and T. Mizutani; Nature, 430, 529 (2004)
5. C. Toyoshima, H. Nomura and T. Tsuda; Nature, 432, 361 (2004)
6. S. Maeda, S. Nakagawa, M. Suga, E. Yamashita, A. Oshima, Y. Fujiyoshi and T. Tsukihara; Nature, 458, 597 (2009)